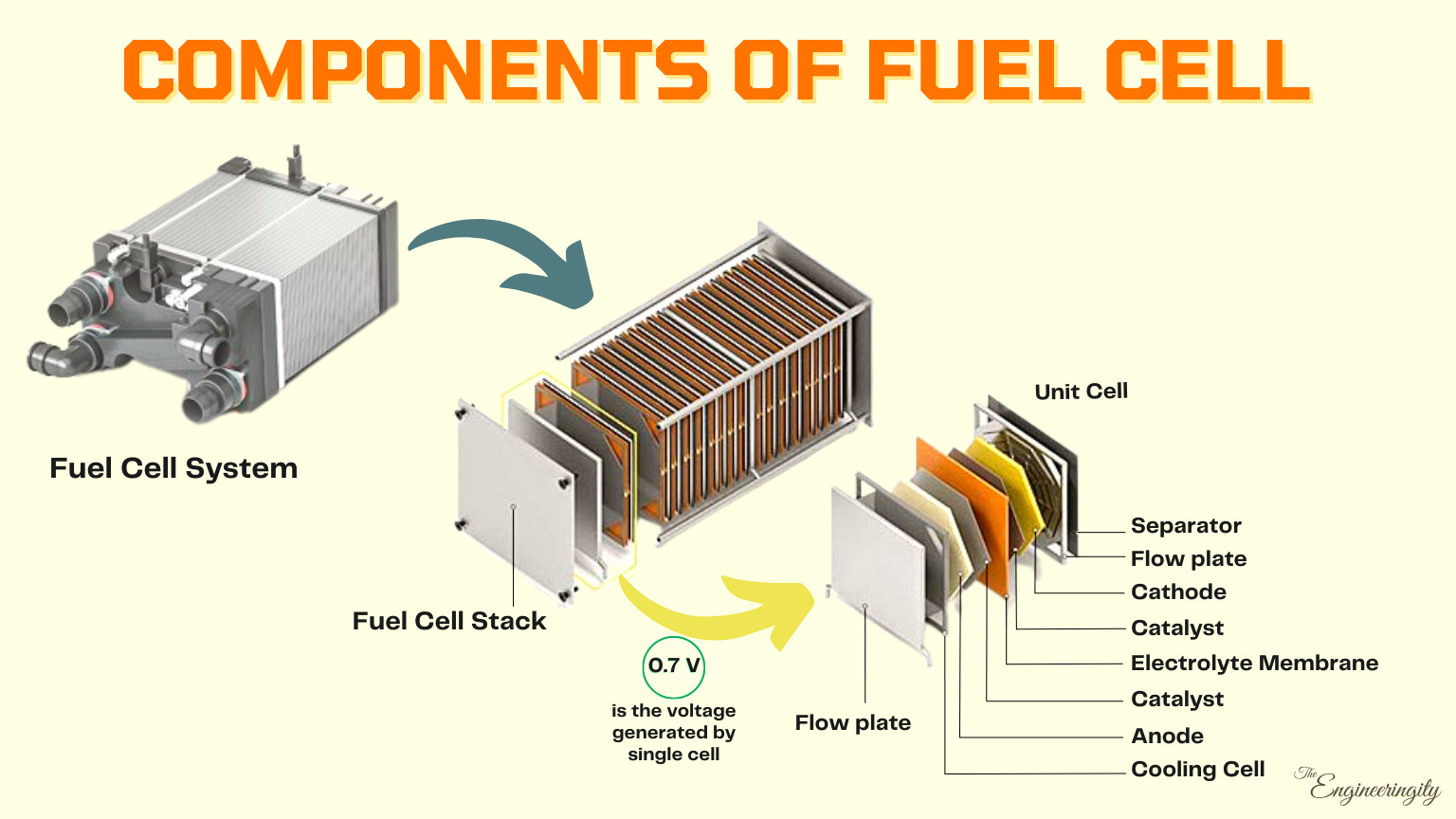
Fuel cell electric vehicles (FCEVs) are powered by hydrogen. One of the major components in FCEVs is the Fuel Cell System (Fuel Stack). The function of the Hydrogen Fuel Cell stack is to drive the motor by producing electricity.
The Fuel cell stack consists of a stack containing up to several hundred fuel cells; it forms the core of the fuel cell system. In each one of these cells arranged in series, a “cold combustion” process takes place that converts the energy from the chemical reaction between the continuously fed hydrogen and airborne oxygen into electricity. This takes place when the hydrogen is catalytically split down into electrons and protons.
Fuel cells have several potential applications, including powering vehicles, providing backup power for homes and businesses, and serving as a source of electricity in remote or off-grid locations. However, they are currently more expensive to produce than traditional fossil fuel-based power sources, and the infrastructure for distributing and storing hydrogen fuel is still under development.
What is a Fuel Cell/ Hydrogen Fuel Cell?
A hydrogen fuel cell is a device that generates electricity by converting the chemical energy of hydrogen fuel into electricity through a chemical reaction with oxygen. Fuel cells are a promising technology for generating electricity because they are relatively efficient, produce very little pollution, and can operate using a variety of fuels, including hydrogen, natural gas, and methanol.
Working of Fuel Cell
In a hydrogen fuel cell, hydrogen gas is fed into the anode (negative electrode), where a catalyst causes the hydrogen atoms to split into protons and electrons. The protons pass through an electrolyte membrane to the cathode (positive electrode), while the electrons are forced to take an alternative path through an external circuit, generating an electric current. At the cathode, the protons and electrons are reunited with oxygen from the air to produce water, the only byproduct of the process. The hydrogen fuel cell is a clean, efficient source of power that produces only water as a byproduct, making it a promising alternative to traditional fossil fuel-based energy sources.
How much energy Hydrogen Fuel Cell can produce?
The fuel cell stack is the heart of a fuel cell power system. It generates electricity in the form of direct current (DC) from electrochemical reactions that take place in the fuel cell. A single fuel cell produces less than 1 V, which is insufficient for most applications. Therefore, individual fuel cells are typically combined in series into a fuel cell stack. A typical fuel cell stack may consist of hundreds of fuel cells. Roughly 400 cells can produce 400 - 500 KW of power. The amount of power produced by a fuel cell depends upon several factors, such as fuel cell type, cell size, the temperature at which it operates, and the pressure of the gases supplied to the cell.
A typical fuel cell produces a voltage from 0.6 to 0.7 V at a full-rated load. Voltage decreases as current increases, due to several factors such as:
Activation loss
Ohmic loss (voltage drop due to resistance of the cell components and interconnections)
Mass transport loss (depletion of reactants at catalyst sites under high loads, causing rapid loss of voltage).
To deliver the desired amount of energy, the fuel cells can be combined in series to yield higher voltage, and in parallel to allow a higher current to be supplied. Such a design is called a fuel cell stack. The cell surface area can also be increased, to allow higher current from each cell.
Components of Hydrogen Fuel Cell
There are several key components in a fuel cell:
Anode:
The anode is the negatively charged electrode where the fuel is introduced. This is the negative terminal of the fuel cell, where the fuel enters the cell and is oxidized. It is typically made of a porous, conductive material that allows the fuel to flow through it and come into contact with the catalysts.
Cathode:
The cathode is the positively charged electrode where the oxidizing agent is introduced. This is the positive terminal of the fuel cell, where the oxidizing agent (usually oxygen) enters the cell and is reduced. It is made of a porous material that allows the oxidizing agent to flow through it and come into contact with the catalysts.
Electrolyte Membrane:
The electrolyte is a thin layer of material that separates the anode and cathode (fuel and oxidant). It allows protons to pass through, but not electrons. It separates the anode and cathode and helps to prevent direct contact between the fuel and oxidizing agent. This creates an electrical potential difference between the anode and cathode, which drives the chemical reaction that generates electricity. For example, in a proton exchange membrane fuel cell (PEMFC), the electrolyte is a proton exchange membrane.
Catalysts:
Catalysts are substances that help to accelerate the chemical reactions that take place at the anode and cathode. They are usually made of precious metals such as platinum or palladium.
Gas diffusion layers:
Gas diffusion layers are porous materials that help to distribute the fuel and oxidizing agent evenly across the surface of the electrodes.
Current collectors:
Current collectors are conductive materials that help to collect the electricity produced by the fuel cell and transmit it to the external circuit.
Bipolar plate:
The bipolar plate is a conductive layer that separates the anode and cathode of the fuel cell. It acts as a conductor, allowing electrons to flow from the anode to the cathode, and it also helps to distribute the fuel and oxidant evenly throughout the cell.
Flow field:
The flow field is a series of channels or grooves etched into the surface of the bipolar plate. It helps to distribute the fuel and oxidant evenly throughout the cell, and it also helps to remove excess heat from the cell.
Cooling system:
Fuel cells generate a lot of heat during operation, so they need a cooling system to keep the temperature within an acceptable range. This is a system that removes heat from the fuel cell to keep it operating at an optimal temperature. This is typically a water-based system, but other materials such as air or a refrigerant can also be used.
Types of Fuel Cell
There are several types of hydrogen fuel cells, including proton exchange membrane fuel cells (PEMFCs), phosphoric acid fuel cells (PAFCs), molten carbonate fuel cells (MCFCs), and solid oxide fuel cells (SOFCs). Each type has its own unique characteristics and is suited for different applications.
There are several different types of fuel cells, each with its own unique characteristics and applications. Some of the main types include:
Polymer Electrolyte Membrane Fuel Cells (PEMFC):
PEM fuel cells use a proton-conducting polymer membrane as an electrolyte. These cells use a polymer membrane as an electrolyte and are often used in portable and portable power applications, such as in vehicles and portable electronics. These are the most commonly used fuel cells in portable and transportation applications. They operate at relatively low temperatures and use hydrogen as fuel.
Alkaline Fuel Cells (AFCs):
Alkaline fuel cells use an aqueous alkaline electrolyte, typically potassium hydroxide, and are known for their high efficiency and long lifespan and are suitable for use in space and military applications. They are often used in large-scale stationary power applications. These fuel cells were some of the first to be developed and are commonly used in space missions due to their high efficiency and ability to operate at low temperatures. They use hydrogen as fuel.
Molten Carbonate Fuel Cells (MCFCs):
Molten Carbonate fuel cells use a molten carbonate electrolyte, typically lithium or sodium carbonate. MCFCs use lithium potassium carbonate salt as an electrolyte, and this salt liquefies at high temperatures, allowing for the movement of charge within the cell – in this case, negative carbonate ions. Like SOFCs, MCFCs are capable of converting fossil fuel to a hydrogen-rich gas in the anode, eliminating the need to produce hydrogen externally. The reforming process creates carbon dioxide emissions. Known for their high efficiency and durability and can operate at high temperatures (around 650°C).
They are suitable for use in stationary power generation such as in power plants and co-generation systems and have high efficiency. They use hydrogen or natural gas as fuel.
Solid Oxide Fuel Cells (SOFCs):
SOFC-type Fuel cells use a solid oxide electrolyte, typically made of ceramics, and can operate at high temperatures (around 1000°C), and are known for their high efficiency and durability. Similar to proton exchange membrane fuel cells and solid oxide fuel cells, they extract electricity from the electrochemical conversion of hydrogen- and oxygen-containing gases, leaving only water as a by-product. Current SAFC systems use hydrogen gas obtained from a range of different fuels, such as industrial-grade propane and diesel. They operate at mid-range temperatures, from 200 to 300 °C. They are suitable for use in stationary power generation and have high efficiency. They can use a variety of fuels, including hydrogen, natural gas, and biofuels.
Phosphoric Acid Fuel Cells (PAFCs):
Phosphoric Acid fuel cells use liquid phosphoric acid as an electrolyte and are suitable for use in stationary power generation and co-generation systems. The electrolyte is highly concentrated or pure liquid phosphoric acid (H3PO4) saturated in a silicon carbide matrix (SiC).
The operating range is about 150 to 210 °C. This high temperature will cause heat and energy loss if the heat is not removed and used properly. This heat can be used to produce steam for air conditioning systems or any other thermal energy-consuming system. Using this heat in cogeneration can enhance the efficiency of phosphoric acid fuel cells from 40 to 50% to about 80%. The electrodes are made of carbon paper coated with a finely dispersed platinum catalyst. They have high efficiency and are relatively easy to maintain. They use hydrogen or natural gas as fuel.
Direct Methanol Fuel Cells (DMFCs):
DFMC fuel cells use methanol as fuel and are suitable for use in portable power applications. They have a high energy density and are relatively easy to refuel.
Hydrogen Economy: The Importance of Fuel Cells in Sustainable Energy Systems
Hydrogen fuel cells have several potential advantages over traditional energy sources. They are highly efficient, with some fuel cells achieving conversion efficiencies of up to 60%. They also produce zero emissions, making them a clean and environmentally friendly source of power. In addition, hydrogen is an abundant, widely available resource, and fuel cells can use a variety of hydrogen sources, including fossil fuels and renewable energy sources such as wind, solar, or hydroelectric power.
The importance of fuel cells in sustainable energy systems lies in their ability to provide clean, efficient power while reducing the world's reliance on fossil fuels. The use of hydrogen as an energy source can help mitigate the negative impacts of climate change by reducing greenhouse gas emissions, improving air quality, and reducing dependence on non-renewable resources.
The transportation sector is one area where fuel cells have shown significant promise. Fuel cell vehicles (FCVs) are already in production, and leading automotive companies are investing heavily in the technology. FCVs have the potential to provide a zero-emission alternative to traditional gasoline and diesel-powered vehicles, with the added benefit of being able to refuel quickly and travel long distances.
In addition to transportation, fuel cells are also being used in stationary power applications, including backup power for critical infrastructure and distributed power generation. Fuel cells can provide reliable power in areas with limited access to traditional power sources, helping to improve energy security and resilience.
The importance of fuel cells in sustainable energy systems cannot be overstated. As the world continues to grapple with the negative impacts of climate change, the use of hydrogen and fuel cells can help to provide a sustainable, reliable, and efficient source of energy. With ongoing research and development, it is expected that fuel cells will play an increasingly significant role in the global transition to a cleaner, more sustainable energy future.
The Economics of Fuel Cells: Cost Analysis and Market Potential
The economics of fuel cells is a critical factor that needs to be considered. In this article, we will explore the cost analysis and market potential of fuel cells.
The cost of fuel cells has been a significant obstacle to their widespread adoption. While the cost of fuel cells has decreased in recent years, they are still relatively expensive compared to traditional energy sources such as fossil fuels. The primary cost drivers for fuel cells include the cost of materials, production processes, and system integration.
In terms of materials, platinum is one of the primary materials used in the production of fuel cells, and its high cost has been a significant factor in the overall cost of fuel cells. Research efforts are currently underway to develop alternative materials that can replace platinum and reduce the cost of fuel cells.
Another significant factor contributing to the cost of fuel cells is the production process. Fuel cells require high-precision manufacturing processes, and this can be time-consuming and expensive. However, as the demand for fuel cells increases, it is expected that economies of scale will bring down the cost of production.
The cost of integrating fuel cell systems into existing infrastructure is also an important consideration. For example, the cost of retrofitting a building with a fuel cell system can be significant, and this can be a significant barrier to adoption.
Despite the high cost, the market potential for fuel cells is significant. The transportation sector, including buses and trucks, is one area where fuel cells have shown promise, and many leading automotive companies are investing in fuel cell technology. Additionally, fuel cells can also be used in stationary power applications, such as powering homes and businesses.
In conclusion, the economics of fuel cells is a critical factor in their widespread adoption. While the cost of fuel cells is still relatively high, ongoing research efforts and economies of scale are expected to bring down the cost of production. As demand for clean and sustainable energy sources continues to grow, fuel cells have the potential to play a significant role in meeting this demand.
Conclusion
However, there are also some challenges to the widespread adoption of hydrogen fuel cells. One major challenge is the cost and availability of hydrogen fuel. Hydrogen must be produced and stored, which can be energy-intensive and expensive. Additionally, hydrogen fuel cells require expensive materials, such as platinum, which can make them expensive to produce.









0 comments
comment down what do you think?